Green Spring Technology's Lutein Encapsulation Technology Boosts Bioavailability
Amid the booming functional food and health supplement industry, a deep-seated technical bottleneck is quietly undermining the fulfillment of product promises: the bioavailability dilemma of active ingredients. This challenge is particularly pronounced in the lutein sector—a globally prevalent eye-health ingredient whose naturally lipophilic properties and molecular instability often limit human absorption efficiency below the critical 30% threshold.
This technical bottleneck triggers a chain reaction: a significant gap exists between consumers' health expenditure and the actual benefits they receive. Market research indicates that over 65% of long-term users question the efficacy of lutein products, while R&D personnel face the dilemma of “formulations being effective but poorly absorbed.” This not only results in tons of raw material waste annually but also erodes the industry'scredibility foundation.
Facing this critical challenge, Green Spring Technology has integrated years of formulation R&D achievements to officially launch a comprehensive range of encapsulated lutein raw material solutions. Through our innovative molecular encapsulation technology system, we have successfully established a new pathway from raw material to absorption, committed to redefining the efficacy standards for lutein products.
This technological breakthrough will lead the industry shift from focusing on “ingredient content” to pursuing “actual absorption,” infusing functional foods with genuine scientific substance. Now, let us jointly initiate this technological revolution from “ingestion” to “absorption,” exploring how to fully unlock the value of every lutein product for consumers.
Part I: How Encapsulation Technology Reshapes Lutein Bioavailability
Based on in-depth research into lutein molecular characteristics, Green Spring Technology has developed a triple-layer encapsulation protection system that systematically addresses bioavailability challenges at the molecular level:
I. Molecular Stability Assurance System
Our patented microencapsulation technology forms a protective barrier around lutein molecules:
·Precise Interface Control: Optimized oil-water interfacial tension achieves ≥95% encapsulation rate
·Enhanced Thermal Stability: Active ingredient retention reaches 92% under high-temperature processing (120°C/30 min)
· Dual Photo-Oxidative Protection: Specialized wall structure reduces photo-oxidative degradation by 40%
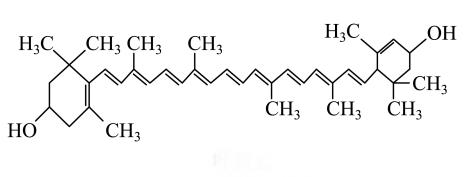
II. Smart Release Technology Platform
Tailored to gastrointestinal conditions, we designed a targeted release mechanism:
1. Gastric Protection Phase
· Encapsulation layer remains stable in gastric acid environment
· Lutein release rate controlled ≤5%
· Effectively prevents gastric acid degradation of active ingredients
2. Intestinal Precision Release
· Triggered by specific intestinal enzyme systems
· Achieves over 85% release within 2 hours
· Synergizes with bile acids to enhance absorption
III. Absorption Enhancement Technology
Through nanoscale particle size control and absorption enhancers:
· Particle Size Optimization: Controls lutein particle size within 100-500nm range
· Permeability Enhancement: Utilizes natural absorption enhancers to increase intestinal permeability
· Lymphatic Transport: Employs lipid carriers to facilitate lymphatic system absorption, bypassing first-pass metabolism
IV. Clinically Validated Data
Confirmed through human trials by third-party authoritative institutions:
· Relative Bioavailability: 2.3 times higher than conventional lutein raw materials
· Peak Plasma Concentration: 2.8 times that of equivalent doses of standard raw materials
· Duration of Action: Extended effective blood concentration maintenance to 8 hours
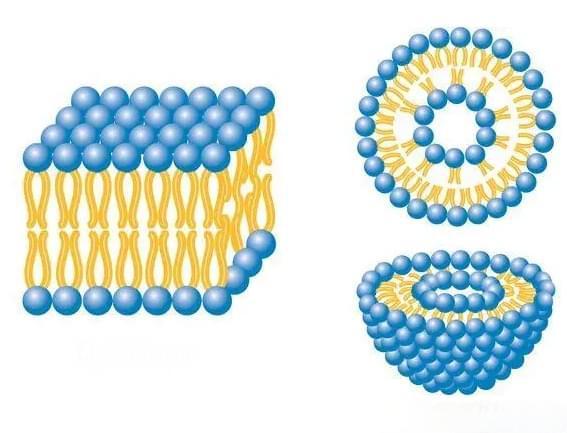
This technological breakthrough not only resolves lutein absorption challenges but also provides innovative approaches for enhancing bioavailability across the functional ingredients industry. Through systematic formulation design, we are redefining efficacy standards for functional components.
Part II: Green Spring Technology's Comprehensive Solutions Tailored to Precise Needs
Leveraging core encapsulation technology, Green Spring Technologies has established a comprehensive four-tier product system delivering specialized solutions for diverse applications:
I. Basic Protection Series: Microencapsulated Lutein Powder
Technical Specifications:
· Encapsulation Efficiency: ≥95% (HPLC method)
· Particle Size Distribution: D50: 50-150μm, Span ≤1.8
· Moisture Content: ≤5% (Karl Fischer method)
· Bulk Density: 0.45-0.55 g/mL
· Angle of Repose: ≤35°
· Shelf Life at Room Temperature: 24 months with ≥90% activity retention
Process Characteristics:
1. Spray-drying process with gum arabic-maltodextrin composite as encapsulation matrix
2. Three-layer encapsulation structure: inner antioxidant protection, middle controlled release, outer physical barrier
3. Thermal stability: withstands processing at 120°C/30 min
4. Excellent compatibility with common excipients (microcrystalline cellulose, lactose, etc.)
Suitable Dosage Forms & Recommended Addition Rates:
· Pressed candies: 0.5–2.0%
· Hard capsules: 1.0–3.0%
· Solid beverages: 0.2–1.0%
· Nutritional powders: 0.5–1.5%
II. Performance Enhancement Series: Water-Dispersible Lutein
Key Technical Parameters:
· Solubility: Rapid dissolution in cold water (complete dispersion within 30 seconds)
· Suspension Stability: Sedimentation rate ≤3% after 15-minute centrifugation at 4000 rpm
· Light Transmittance: ≥85% (at 650nm wavelength)
· Zeta Potential: ≥-30mV
· Emulsion Stability: No separation at 85°C/30min
Innovative Breakthroughs:
1. Utilizes high-shear emulsification coupled with spray drying technology
2. Utilizes modified starch-phospholipid composite emulsification system
3. Broad pH adaptability (3.0-8.0)
4. Excellent compatibility with sweeteners, acidulants, and preservatives
Application Examples:
· Functional beverages: 0.01-0.05% (transparency retention)
· Sports drinks: 0.02-0.08% (electrolyte compatibility)
· Oral liquids: 0.05-0.1% (long-term stability)
· Dairy products: 0.03-0.06% (protein compatibility)
III. Premium Enhancement Series: Nanoscale Lutein Formulation
Precision Technical Specifications:
· Average particle size: 80-100nm (Dynamic Light Scattering)
· Polydispersity index: 0.15-0.25
· Encapsulation rate: ≥98%
· Bioavailability: 150-200% higher than standard raw materials
· Cellular studies demonstrate: 180% increased uptake by retinal pigment epithelial cells
Core technological advantages:
1. Nano-scale processing via combined high-pressure homogenization and ultrasonication
2. Liposome-polymer hybrid delivery system
3. Dual-response release mechanism (temperature/pH)
4. In vitro simulated digestion model validation demonstrates significantly enhanced intestinal absorption
Target Market Positioning:
· Pharmaceutical-grade health supplements: For macular degeneration prevention
· Professional sports nutrition: Rapid absorption formulations
· Infant nutrition: High bioavailability formulations
· Premium functional foods: Differentiated competitive products
IV. Specialized Functional Series: Customized Solutions
Personalized Service Offerings:
1. Synergistic Compound Formulations
· Lutein + Zeaxanthin (5:1 golden ratio)
· Lutein + Anthocyanin synergistic formulation
· Lutein + Omega-3 composite system
2. Controlled-Release Formulations
· 12-hour sustained-release formula
· Gut-targeted release system
· Dual-layer release technology (immediate + sustained release)
3. Specialized Applications
· Baking-Specific (200°C Heat Resistance)
· Candy-Specific (High Shear Tolerance)
· Dairy-Specific (Fermentation Stability)
V. Quality Assurance & Service System
Standardized Production System:
· GMP & ISO22000 Dual-Certified Production Base
· Online Quality Monitoring (PAT Technology)
· Batch-to-Batch Consistency Control (RSD≤3%)
· Full-process traceability system
Technical Support Commitment:
· 24-hour technical response
· Free sample customization service
· Open application laboratory
· Joint development project support
Through this comprehensive product matrix, Green Spring Technology delivers optimal lutein solutions tailored to clients at diverse development stages and market positions. We look forward to collaborating with industry partners to advance technological progress and innovation in the functional food sector.
Part III: Applications of High Bioavailability Lutein Ingredients
I. Application Case Studies
1. Functional Beverage Development Case
A renowned brand adopted water-dispersible ingredients:
· Shelf-life stability: >95% content retention over 12 months
· Consumer acceptance: 32% improvement in taste ratings
· Market performance: 18% market share within 6 months of launch
2. Health Supplement Upgrade Case
Traditional tablets replaced with microencapsulated raw materials:
· Product differentiation: Established technological barriers
· Average order value increase: 45%
· Repurchase rate growth: 28%
II. Quality Assurance System
1. Standardized Quality Control
· Monitoring of 57 quality indicators
· Real-time process analysis technology
· Full lifecycle quality traceability
2. Stability Study Data
Accelerated testing results demonstrate:
· 6 months at 40°C/75% RH
· Content retention rate: 98.2%
· Related substance increase: ≤0.5%
Green Spring Technology's encapsulated lutein raw materials not only deliver technologically advanced solutions but also create significant market value and competitive advantages for our clients. We look forward to establishing long-term, mutually beneficial partnerships with industry peers through these reliable data and case studies.
Part IV: Initiating Collaboration
Our Commitment:
· Complimentary technical consultation and sample provision
· Customized formulation solutions
· Comprehensive technical support throughout the process
Póngase en contacto con nosotros
Phone: +86 29 88313578
WhatsApp: +86 13649243917
Email: helen@greenspringbio.com
Website: https://www.greenspringnatural.com/
Service Commitments
· 2-hour response time for inquiries
· Sample delivery within 3 business days
· Professional multilingual technical support
Contact us now for free samples and technical solutions!
Let’s collaborate to create more competitive products and build a successful future together.
Referencias:
[1] Liu Yang, Chen Mingjun, Song Xiang, et al. Efecto de la luteína sobre el grado de respuesta inflamatoria en placas ateroscleróticas de la arteria caró[J]. Food Science, 2018, 39(9): 170-175.
[2]ABDEL-AAL E M, AKHTAR H, ZAHEER K, et al. Fuentes dietéticas de carotenoides de luteína y zeaxantina y su papel en la salud ocular [J]. Nutrientes, 2013, 5(4): 1169-1185. DOI:10.3390/nu5041169.
[3] Hou Yanmei, Wu Tong, Xie Kui. Avance de la investigación sobre la actividad biológica de la luteína [J]. China Food and Nutrition, 2020, 26(10): 5-8. DOI:10.19870/j.cnki. 11-3716/ ts.20200902.001.
[4]BECERRA M O, CONTRERAS L M, LO M H, et al. La luteína como ingrediente alimentario funcional: estabilidad y biodisponibilidad [J]. Journal of Functional Foods, 2020, 66: 103711. DOI:10.1016/j.jff.2019.103771.
-
anterior
Water-Soluble Lutein Ingredient Empowers Clear Beverages
-
siguiente
Fresh Spirulina | High Beta-Carotene Content | The Choice for an Enhanced Formula


 inglés
inglés francés
francés español
español ruso
ruso coreano
coreano Japonés japonés
Japonés japonés




